Kenya to roll out lenacapavir HIV prevention injection by 2026 in landmark move
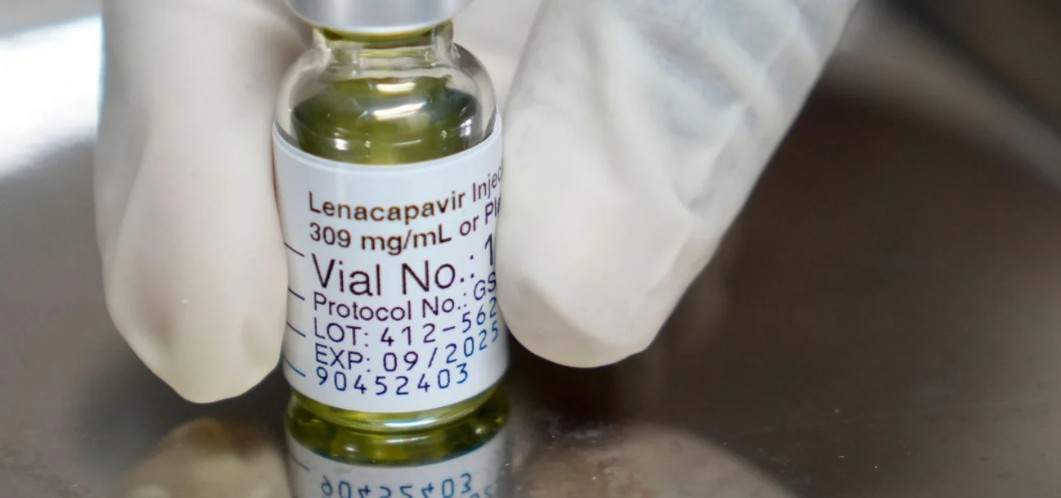
The injectable drug, approved by the US Food and Drug Administration in June 2025 and added to updated World Health Organisatio guidelines in July 2025, will complement existing pre-exposure prophylaxis (PrEP) methods.
Kenya is among eight African countries set to pioneer the rollout of lenacapavir, a new long-acting injectable drug for HIV prevention, aimed at reducing infections, particularly among young people.
Appearing before the Senate Standing Committee on Labour on Tuesday, Health Cabinet Secretary Aden Duale said the drug—administered just twice a year—will be available in Kenya by January 2026.
More To Read
- WHO approves injectable lenacapavir in major shift on HIV prevention
- World Zoonoses Day: Scientists warn Kenya faces rising threat from animal-borne diseases
- Nairobi residents urged to donate more blood amid 30 per cent shortfall
- MoH flags surge in youth HIV infections, calls for stronger prevention strategies
- Kenya marks World Blood Donor Day as state, Red Cross urge donations to save lives
- WHO urges HIV testing for all mpox patients as global cases top 129,000
“Kenya remains steadfast in its commitment to ending the HIV epidemic through innovative, people-centred approaches,” Duale said.
He noted that Kenya's national HIV prevalence stands at 3.7 per cent, with approximately 1.4 million people living with the virus. He added that 41 per cent of new infections are occurring among young people under the age of 24.
“The urgency for impactful and tailored solutions has never been greater,” he said.
Kenya is among nine countries chosen to roll out Lenacapavir, alongside Eswatini, Lesotho, Mozambique, Nigeria, South Africa, Uganda, Zambia, and Zimbabwe.
Long-term HIV prevention
Duale said the move marks a major step in expanding access to discreet and long-term HIV prevention options across the region.
The injectable drug, approved by the US Food and Drug Administration (FDA) in June 2025 and added to updated World Health Organisation (WHO) guidelines in July 2025, will complement existing pre-exposure prophylaxis (PrEP) methods.
“Administered just twice a year, Lenacapavir represents a paradigm shift in HIV prevention, especially for populations vulnerable to stigma and adherence challenges,” he said.
Describing the drug as a “biomedical breakthrough,” Duale said its integration into Kenya’s national HIV response strategy reflects the government’s commitment to equity, innovation and community-led health solutions.
“This marks a significant milestone in Kenya’s fight against HIV, offering individuals at substantial risk a highly effective and discreet alternative to daily oral PrEP,” he said.
He added that the Ministry of Health has already developed an implementation plan and, through a consultative process with stakeholders, is finalising national guidelines to facilitate a smooth rollout.
“Through NASCOP and our partners, we are actively working to ensure this innovative product is accessible to Kenyans by January 2026,” Duale said.
He emphasised that the ministry is mobilising necessary systems and resources to ensure the timely availability of the intervention, while calling on stakeholders to align efforts and support the initiative.
“We remain committed to ensuring equitable access, strengthening community engagement, and integrating Lenacapavir into our national policies to reach priority populations effectively,” Duale said.
“We call upon all stakeholders to align efforts, enhance delivery platforms, and support this transformative journey. Together, we are forging a path toward a future free of HIV.”
Top Stories Today
Reader Comments
Trending