Pharmacy and Poisons Board flags falsified batch of Avastin (Bevacizumab 100 mg)
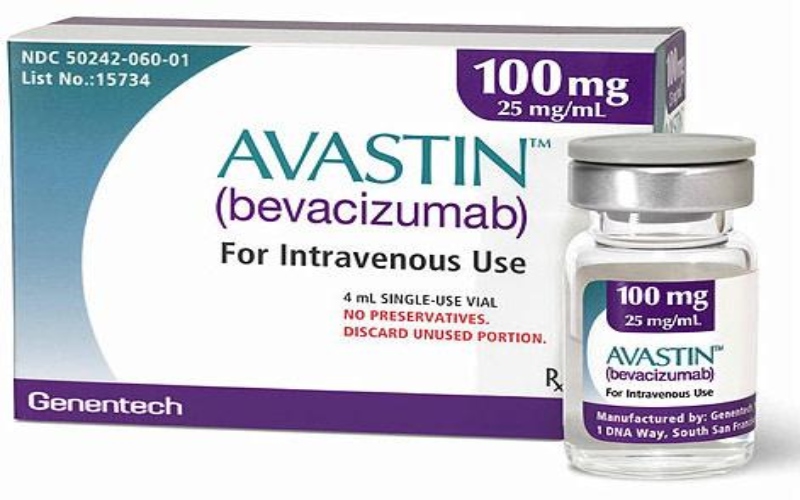
Avastin (Bevacizumab) is a prescription-only medication used in the treatment of various types of cancers, including colorectal cancer, lung cancer, kidney cancer, cervical cancer, and certain types of eye conditions such as age-related macular degeneration (AMD).
The Pharmacy and Poisons Board (PPB) has identified a falsified batch of Avastin (Bevacizumab 100 mg) injection, Batch Number H0573B01, falsely purporting to be manufactured by Roche, currently circulating in the Kenyan market.
Avastin (Bevacizumab) is a prescription-only medication used in the treatment of various types of cancers, including colorectal cancer, lung cancer, kidney cancer, cervical cancer, and certain types of eye conditions such as age-related macular degeneration (AMD). The integrity and authenticity of this drug are critical to ensuring patient safety and treatment efficacy.
In a statement released on Thursday, Dr A. Mohamed, the acting Chief Executive Officer of the PPB, urged all stakeholders to remain vigilant, emphasising the urgency and seriousness of the situation.
More To Read
- Kenya raises alarm on surge in synthetic drugs, shifting trafficking trends
- Pharmacy board dismisses claims of medicine import ban, says drug supply uninterrupted
- Drug shortage fears mount as distributors push for reinstatement of 21,000 blocked medicines
- Pharmacy and Poisons Board assures paracetamol use during pregnancy safe
- Pharmacy board warns Kenyans against off-label use of Ozempic for weight loss
- Senators order crackdown on absenteeism in public hospitals
The Board directed all procurement agencies, distributors, pharmacists, pharmaceutical technologists, healthcare workers, and members of the public to be on high alert and immediately report any suspected cases involving the falsified batch of Avastin.
"All stakeholders in the pharmaceutical supply chain are strongly advised to source Health Products and Technologies (HPTs) exclusively from licensed manufacturers, importers, distributors, and retailers. Obtaining products from unlicensed sources endangers patient safety and will attract strict regulatory and legal consequences," the Board stated.
It added that it will, in collaboration with investigative agencies, initiate legal and regulatory actions against any individual or entity found to be involved in the distribution or circulation of this falsified batch, as per the provisions of the Pharmacy and Poisons Act (CAP 244).
Surge in counterfeit drugs
The circulation of counterfeit and substandard medicines is alarmingly on the rise in Kenya, with many being sold through illegal markets and to unsuspecting consumers.
Estimates indicate that these falsified and poor-quality drugs may now account for up to 30 per cent of Kenya's approximately Sh15 billion pharmaceutical market.
These substandard products often contain little or no active ingredients, and in some cases, may include harmful contaminants, putting patients at serious risk.
A study revealed that 22.6 per cent of the 669 antimicrobial samples tested across the region failed at least one quality assessment.
The situation is even more concerning in Kenya, where nearly 38 per cent of commonly used antibiotics, such as amoxicillin and co-trimoxazole, failed quality tests. These antibiotics are widely prescribed, particularly for children and for treating common infections.
Failures identified in these medicines include insufficient active ingredients and poor dissolution rates, which compromise treatment effectiveness and contribute significantly to the growing crisis of antimicrobial resistance (AMR).
Top Stories Today