Lenacapavir: A promising breakthrough for HIV prevention in pregnant, breastfeeding women
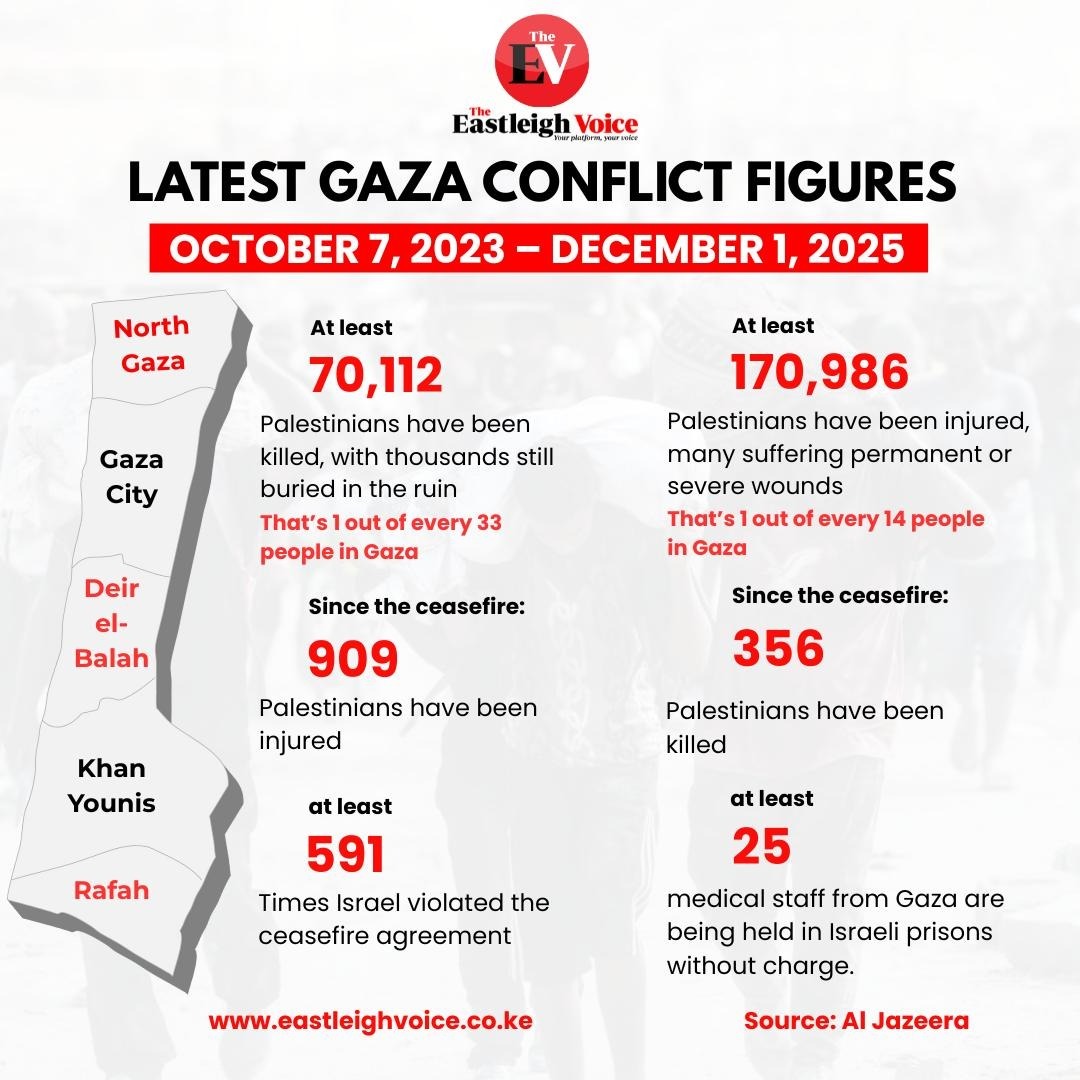
In 2024, 20,105 adults were newly infected with HIV - a sharp rise from 3,353 cases recorded in 2023. Infections among children also surged from 606 to 4,349 during the same period.
Mother-to-child transmission of HIV remains a critical challenge as new data reveal a worrying increase in infections across the country in 2024.
According to statistics released by the National Syndemics Disease Control Council (NSDCC), adults and children have been affected, signalling a significant setback after three years of steady decline.
More To Read
- German researchers find highly effective HIV antibody
- Zambia, Eswatini first in Africa to receive twice-yearly HIV prevention injection as Kenya eyes approval
- Tanzanian woman's immune response yields antibody capable of halting most HIV variants
- Zambia approves injectable HIV prevention drug
- South Africa becomes first African nation to offer twice-yearly HIV prevention shot
- 'We feel forgotten': People living with HIV decry stigma as US aid cuts bite
In 2024, 20,105 adults were newly infected with HIV, a sharp rise from 3,353 cases recorded in 2023. Infections among children also surged from 606 to 4,349 during the same period. Women accounted for the majority of the new cases, with 13,236 infections reported, double the number recorded among men.
In a recent correspondence published in The Lancet, global health experts are urging governments, especially those in regions most affected by HIV, to make pregnant and breastfeeding women a top priority in the roll-out of lenacapavir (LEN), a new long-acting injectable used for HIV prevention (PrEP).
LEN has the potential not only to protect women during pregnancy and breastfeeding but also to prevent mother-to-child transmission of HIV, which remains a major public health challenge in many parts of the world.
Despite being at very high risk of both acquiring HIV and passing it on to their babies, pregnant and lactating women are often left out of HIV prevention efforts. This gap in protection has devastating consequences.
 A photo of a pregnant woman. Pregnant and lactating women are often left out of HIV prevention efforts. (Photo: Freepik)
A photo of a pregnant woman. Pregnant and lactating women are often left out of HIV prevention efforts. (Photo: Freepik)
For example, in Kenya, over 7 per cent of babies born to women living with HIV in 2023 were infected, mainly due to vertical transmission - when HIV is passed from mother to child during pregnancy, birth, or breastfeeding. Globally, an estimated 120,000 children acquired HIV in 2024, with most cases occurring in southern Africa.
The Purpose-1 trial marked an important shift by including pregnant and breastfeeding women from the start. It showed that lenacapavir is safe for mothers and their babies, paving the way for more inclusive research and practical use. However, countries like Kenya have not yet begun offering LEN, and there is an urgent need to close this gap.
They propose a strategy called "two for two", a simple and potentially game-changing approach: just two injections of lenacapavir during pregnancy and postpartum could protect both the mother and her baby for up to a year, covering the periods when the risk of HIV transmission is highest.
To make LEN widely accessible, they call for it to be integrated into existing maternal health services, such as antenatal care, child vaccination clinics, and family planning programs. Ensuring that women have a choice, whether LEN, oral PrEP, the vaginal ring, or condoms, is key to an effective and equitable HIV prevention strategy.
A clinical case report published in the Journal of Antimicrobial Chemotherapy in December 2024 described the use of lenacapavir during pregnancy in a 31-year-old woman with multidrug-resistant HIV and a long history of poor adherence to oral antiretroviral therapy
Despite efforts to support adherence early in pregnancy, her viral load remained high, around 52,000 copies/mL, into the third trimester. At approximately 27 weeks of gestation, a multidisciplinary team initiated a long-acting injectable regimen consisting of lenacapavir, enfuvirtide, and ibalizumab.
 HIV test kit. In 2024, 20,105 adults were newly infected with HIV. (Photo: Freepik)
HIV test kit. In 2024, 20,105 adults were newly infected with HIV. (Photo: Freepik)
Lenacapavir was administered subcutaneously at 928 mg following an oral lead-in. One week after the injection, her viral load dropped sharply to 542 copies/mL, indicating rapid viral suppression. However, a few weeks later, a viral rebound occurred.
Genotypic testing revealed an N74D capsid mutation, confirming the emergence of resistance to lenacapavir. This resistance likely developed because lenacapavir was used without full, effective companion drugs during the initial phase - creating a window of functional monotherapy.
At delivery, no HIV transmission to the infant occurred. Lenacapavir was detected in the infant's plasma on day 3 of life (30.60 µg/L), confirming that it crosses the placenta. The drug levels in the infant declined steadily and were below 5 ng/mL by 1, 3, and 6 months of age. The baby showed no adverse effects during six months of follow-up.
This case demonstrates that lenacapavir can be absorbed during pregnancy, can cross the placenta, and can contribute to rapid viral suppression. However, it also highlights the significant risk of resistance development when used without adequate companion drugs. While the infant remained HIV-negative and healthy, the report emphasises that this is a single case, and results should not be generalised.
Lenacapavir is set to become significantly more affordable in low- and middle-income countries, a move health experts say could be a turning point in the global fight against HIV/AIDS. The twice-yearly injectable, which currently costs $28,218 (approximately Sh3.6 million) per person annually, will now be offered for just $40 (Sh5,172) under a new pricing agreement.
Clinical trials have shown remarkable results, with one study reporting a 100 per cent success rate in preventing HIV infection. The drug received endorsement from the World Health Organisation (WHO) in July and has already been approved by regulators in the United States and Europe.
Top Stories Today