South Africa recalls Benylin cough syrup days after Kenya halts sale
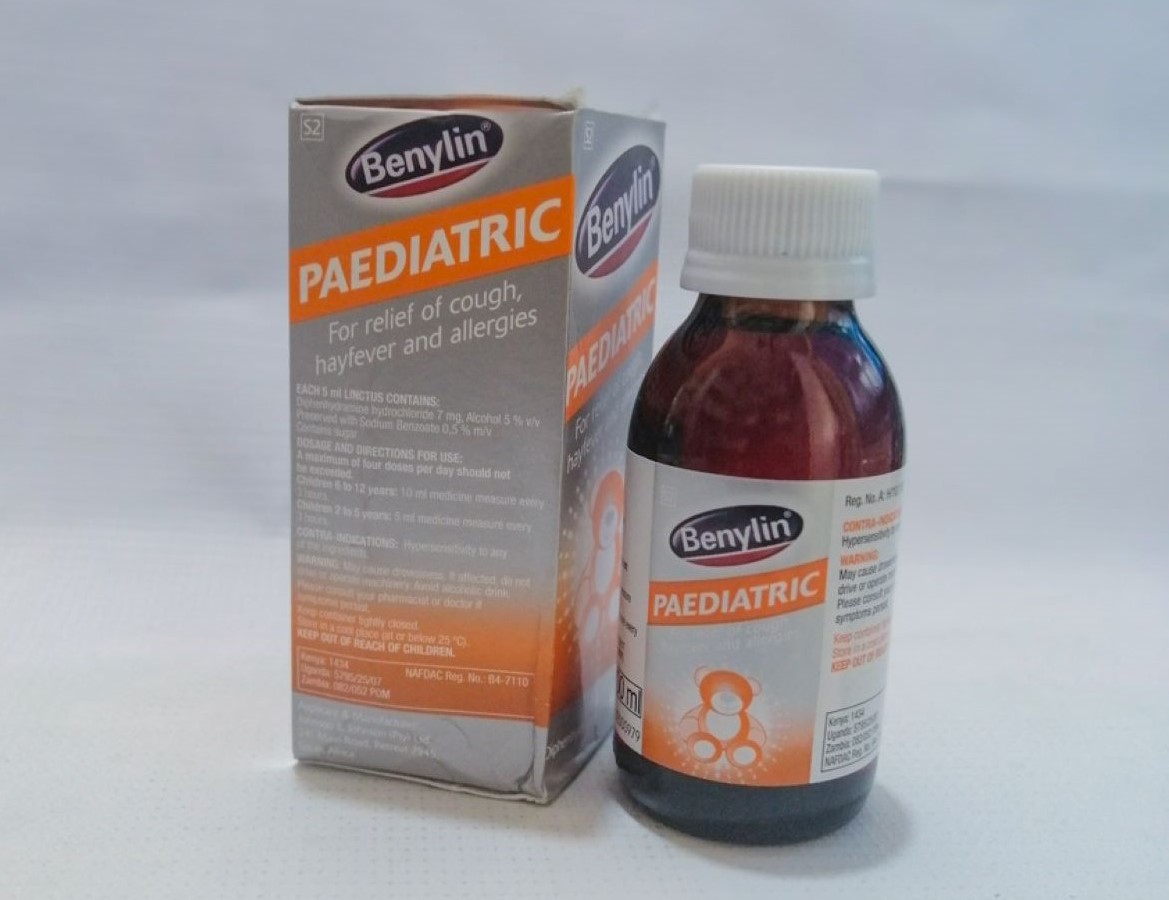
The affected batches were sold in six countries including Kenya, South Africa, Eswatini, Rwanda, Tanzania and Nigeria.
South Africa's health regulator has recalled the Johnson & Johnson cough syrup days after Kenya halted its sale in the market.
The recall follows a report by the regulator's Nigerian counterpart on Wednesday, which first detected diethylene glycol toxin in the Benylin Paediatric 100-ml cough syrup.
More To Read
- Government orders supermarkets, retailers to cease selling unregistered and counterfeit medicines
- Pharmacy Board urges Kenyans to report any adverse drug reactions amid spike in cases
- Government orders crackdown on counterfeit and poor-quality medicines in Kenya
- African nations pledge to boost local manufacturing of medical products
- Kenya leads push for stronger regulation of medicines, vaccines in Africa
- KMPDC launches 2026 licence renewal for doctors, health facilities
The affected batches were sold in six countries including Kenya, South Africa, Eswatini, Rwanda, Tanzania and Nigeria.
The syrup is used in the treatment of hay fever and other allergic conditions affecting the upper respiratory tract.
In a statement on Thursday, Kenya's Pharmacy and Poisons Board (PPB) chief executive officer Fred Siyoi said diethylene glycol is a contaminant which is toxic to humans when consumed and can prove fatal.
"Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state, and acute kidney injury, which may lead to death,” Siyoi warned.
He said that the batch under investigation was manufactured in May 2021, with an expiration date set as April 2024.
The public was also advised to return the product to the nearest healthcare facility, while healthcare facilities are advised to return the products to the respective suppliers.
Seek medical attention
Patients, including children who had consumed the product, were also urged to immediately seek medical attention from a qualified healthcare professional.
PPB assured the public that they will establish mechanisms to ensure that medicines supplied to the Kenyan market meet the required Quality, Safety and Efficacy standards.
"We encourage the public to be vigilant at all times and report any suspected poor-quality medicines or adverse drug reactions to the nearest healthcare facility and the Pharmacy and Poisons Board,” Siyoi said.
Kenvue (KVUE.N) which now owns the Benylin brand after a spin-off from J&J last year, said in a statement that it is conducting its own assessment and working with health authorities to determine a course of action.
"A review of our global safety database for the period between product release in May 2021 and up to 11 April 2024 did not identify any serious adverse events for any batch of Benylin Paediatric Syrup," it said.
High levels of diethylene glycol in cough syrup have been linked to the deaths of dozens of children in Gambia, Uzbekistan and Cameroon since 2022 in one of the world's worst waves of poisoning from oral medication.
Top Stories Today











































