Pharmacy Board raises alarm over mishandling of medical products

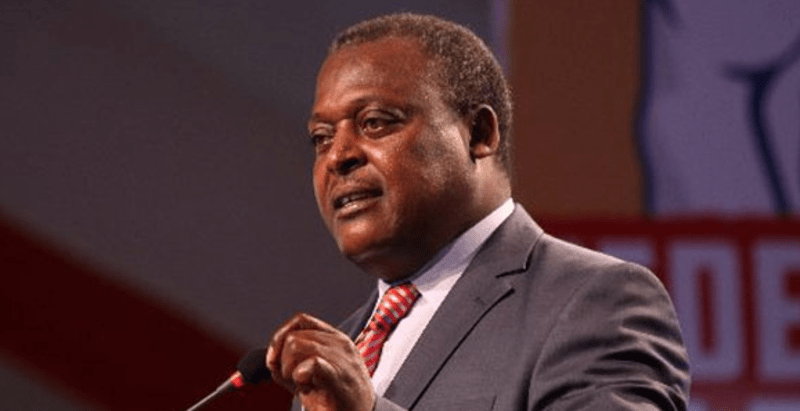
In a statement on Wednesday, the board’s CEO Fred Siyoi PPB emphasised the need for strict adherence to manufacturer-recommended temperature and humidity levels throughout the supply chain.
The Pharmacy and Poisons Board (PPB) has raised concerns over the improper storage and handling of medical products, warning that such practices are putting patient health at risk.
The regulator has received multiple complaints about the degradation of Health Products and Technologies (HPTs), which it attributes to failure to follow proper storage conditions.
More To Read
- Out-of-pocket medicine costs surge amid drug shortages in Kenya
- Pharmacy Board urges Kenyans to report any adverse drug reactions amid spike in cases
- African nations pledge to boost local manufacturing of medical products
- Kenya leads push for stronger regulation of medicines, vaccines in Africa
- Africa imports over 70 per cent of its medicines — Local production of ingredients could change that
- KMPDC launches 2026 licence renewal for doctors, health facilities
In a statement on Wednesday, the board’s CEO Fred Siyoi PPB emphasised the need for strict adherence to manufacturer-recommended temperature and humidity levels throughout the supply chain.
“All manufacturers, distributors, wholesalers, retailers, and healthcare facilities must store and handle HPTs according to manufacturer-specified temperature and humidity conditions,” he said.
The board urged industry players to follow Good Distribution Practices (GDP) to prevent medicines and other health supplies from losing their quality before they reach patients.
It directed manufacturers to ensure that all HPT formulations meant for distribution in Kenya can withstand the country’s high temperatures and humidity.
“Manufacturers must ensure that any HPT formulation intended for distribution in Kenya is designed, developed, and tested to withstand high-temperature and high-humidity environmental conditions,” Siyoi said.
To strengthen compliance, the board outlined specific measures that marketing authorisation holders must follow.
These include conducting stability studies to confirm product safety and efficacy, using packaging that protects against environmental conditions, and clearly labeling storage instructions.
“Marketing authorisation holders must conduct stability studies in accordance with ICH Zone IVb climatic conditions to ensure the formulation maintains quality, safety, and efficacy throughout its shelf life,” Siyoi stated.
It also called for the proper training of supply chain stakeholders to prevent temperature-sensitive products from being mishandled.
PPB announced that it will be carrying out inspections and compliance audits to ensure the new directives are followed.
“Non-compliance may result in regulatory action, including product recalls, license suspension, or other enforcement measures,” Siyoi warned.
The board urged all stakeholders to take immediate steps to meet the new requirements to ensure the safety and effectiveness of medicines and other health-related products in the country.
Top Stories Today