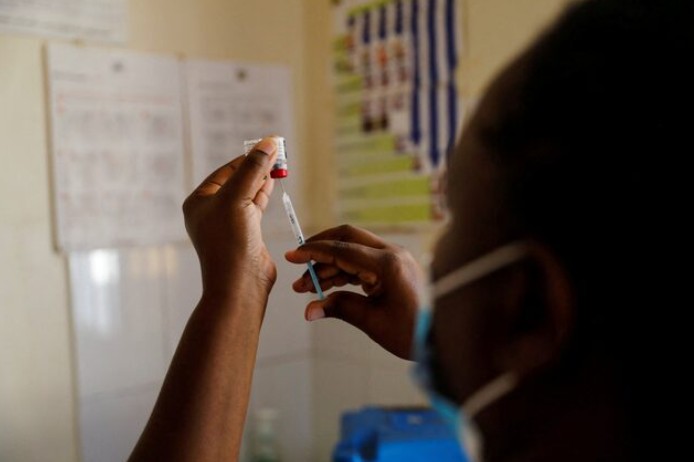
First malaria drug for infants approved, to be rolled out in Africa within weeks
Eight African countries participated in clinical trials of the drug and are expected to be among the first to access it.
A new malaria drug specifically designed for newborns and infants has been approved for use, closing a critical treatment gap for a group previously left without targeted medication.
The drug, known as Coartem Baby, was developed by pharmaceutical company Novartis in partnership with the Medicines for Malaria Venture (MMV), a Swiss-based non-profit.
More To Read
- Undocumented status, ID barriers hamper vaccine access for vulnerable mothers, children in Kenya
- Kenya receives 3 million BCG doses as Ministry of Health races to end vaccine shortage
- Invasive urban mosquito threatens to worsen malaria in Kenya, scientists warn
- Mombasa begins countywide fumigation to curb Chikungunya outbreak
- Vaccines: Why these young Africans are hesitant about them and what might change their minds
- Fear, misinformation plague parents despite child vaccines shortage resolution
The medication, approved by Swiss regulators, is intended for infants weighing under 4.5kg and will be distributed on a largely not-for-profit basis in countries with the highest malaria burden.
Eight African countries participated in clinical trials of the drug and are expected to be among the first to access it.
This marks the first time a malaria treatment has been specifically formulated and approved for babies. Until now, infants have been treated with modified versions of drugs meant for older children, putting them at risk of overdose and serious organ complications.
"For more than three decades, we have stayed the course in the fight against malaria, working relentlessly to deliver scientific breakthroughs where they are needed most," Novartis CEO Vas Narasimhan said on the development, according to BBC.
"Together with our partners, we are proud to have gone further to develop the first clinically proven malaria treatment for newborns and young babies, ensuring even the smallest and most vulnerable can finally receive the care they deserve."
The Novartis CEO's sentiments were shared by his MMV counterpart Martin Fitchet who lauded the development as a major breakthrough in saving the lives of newborns and infants.
"Malaria is one of the world's deadliest diseases, particularly among children. But with the right resources and focus, it can be eliminated," he said.
"The approval of Coartem Baby provides a necessary medicine with an optimised dose to treat an otherwise neglected group of patients and offers a valuable addition to the antimalarial toolbox."
According to the World Health Organisation (WHO), Africa continues to bear the brunt of the global malaria burden.
In 2023, African Union member states accounted for 251 million malaria cases, 95 per cent of all global infections, and over 579,000 deaths, representing 97 per cent of malaria-related fatalities.
Alarmingly, 76 per cent of these deaths were among children under the age of five. An estimated 1.3 billion people across the continent remain at risk, with 192 infections per 1,000 people and 44 deaths per 100,000.
Despite this, the region has made progress compared to 2000, with malaria infections down by 34 per cent and deaths reduced by 61 per cent.
Kenya has also made strides in tackling the disease, cutting its malaria prevalence from 8.2 per cent in 2015 to 6 per cent in 2023.
In June 2024, Kenya secured a $72.9 million (Sh9.4 billion) grant from the Global Fund to support malaria programs from July 2024 to June 2027, to reduce cases and deaths by 75 per cent during that period.
Top Stories Today