Pharmacy board warns Kenyans against off-label use of Ozempic for weight loss
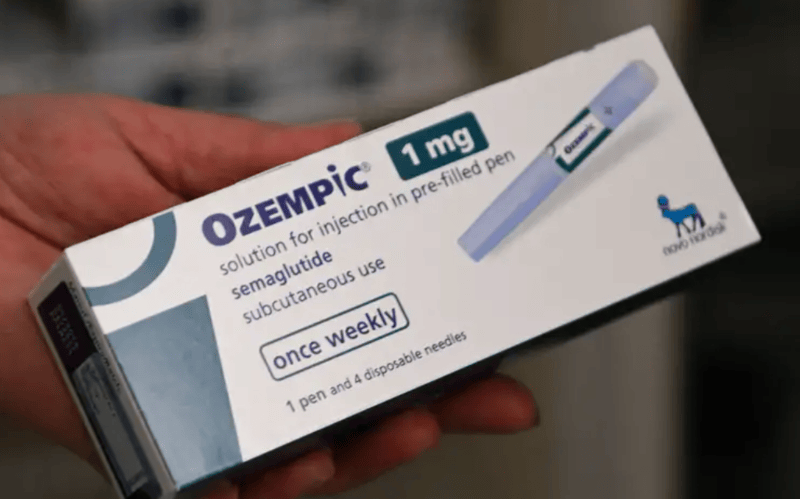
The drug has increasingly been used for weight loss across the country, with reports also emerging about counterfeit versions circulating in the market.
The Pharmacy and Poisons Board (PPB) has warned Kenyans against the use of off-label semaglutide compounds, also known as Ozempic, for weight loss, saying the drug is strictly a Prescription-Only Medicine whose misuse could lead to serious health complications.
In a statement on Tuesday, noted that although the medicine is effective in managing type 2 diabetes that is insufficiently controlled, its use outside approved medical purposes continues to raise safety concerns.
More To Read
- Ozempic and other weight-loss drugs linked to rare but serious eye conditions
- Study finds patients quickly regain weight after coming off Ozempic
- Senators order crackdown on absenteeism in public hospitals
- How fake medication is a problem across the world
- Public hospital patients may have received untested drugs, Auditor-General reveals
- Beyond chlorine: Why your eyes turn red after swimming
“Semaglutide is a prescription-only medicine and should not be used without medical supervision. It is approved for the treatment of adults with Type 2 diabetes mellitus that is insufficiently controlled. While the benefits of semaglutide outweigh its risks, serious safety concerns continue to be raised, particularly when it is used outside its approved medical purposes,” the Board said.
The PPB explained that semaglutide, commonly marketed to the public under brand names such as Ozempic and other generic names, can lead to side effects ranging from mild to severe. Some of the most common risks include low blood sugar, also known as hypoglycemia, eye conditions, acid reflux disease and intestinal obstruction.
The Board urged members of the public to refrain from off-label use of the drug and encouraged them to report suspected side effects or poor-quality medicines through its official reporting channels.
These include the Pharmacovigilance Electronic Reporting System available at pv.pharmacyboardkenya.org, email via [email protected], telephone on +254 795 743 049, or through the public USSD code *271#.
“The Board appreciates your continued support and collaboration in promoting the safe use of medicines,” it said.
The drug has increasingly been used for weight loss across the country, with reports also emerging about counterfeit versions circulating in the market.
Public Health Principal Secretary Mary Muthoni recently addressed concerns over the misuse of Ozempic in Kenya and confirmed that swift regulatory actions are underway to protect consumers and ensure that all pharmaceuticals are prescribed and dispensed responsibly.
Top Stories Today