Zambia approves injectable HIV prevention drug
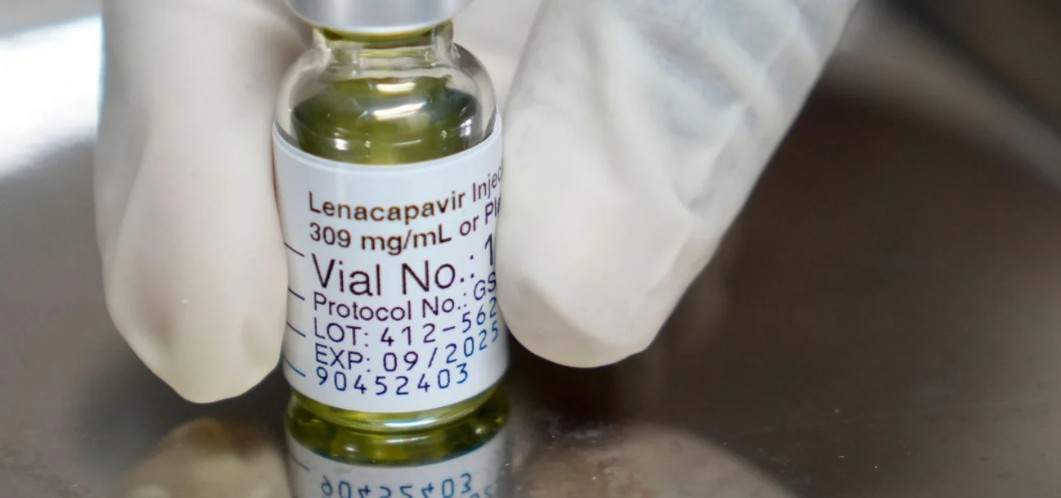
Lenacapavir is administered once every six months and offers protection against HIV infection for the same duration.
Zambia has approved Lenacapavir, a long-acting injectable antiretroviral (ARV) drug, for the prevention of HIV/AIDS, with the government describing it as a major milestone in the country's fight against the pandemic.
Minister of Health Elijah Muchima said on Friday that the approval of the drug reaffirms the government's commitment to ending HIV as a public health threat by 2030 through the introduction of innovative and highly effective prevention technologies.
More To Read
- German researchers find highly effective HIV antibody
- Zambia, Eswatini first in Africa to receive twice-yearly HIV prevention injection as Kenya eyes approval
- Tanzanian woman's immune response yields antibody capable of halting most HIV variants
- Viral infections linked to higher risk of heart attacks and strokes
- South Africa becomes first African nation to offer twice-yearly HIV prevention shot
- Lenacapavir: A promising breakthrough for HIV prevention in pregnant, breastfeeding women
Lenacapavir is administered once every six months and offers protection against HIV infection for the same duration.
According to Muchima, the registration of the drug makes Zambia the second country in Africa, after South Africa, to do so.
He noted that the introduction of the drug has significant implications for HIV prevention in Zambia, which continues to record about 30,000 new infections annually, particularly among adolescent girls and young women.
"The availability of this drug gives hope to those who struggle to take daily or two-monthly HIV prevention doses, pregnant and breastfeeding mothers at risk of infection, and men who prefer to access prevention privately," he said.
Muchima said the milestone builds upon the country's introduction of another long-acting injectable ARV, Cabotegravir, in February 2024, adding that Lenacapavir will be provided free of charge to all eligible Zambians.
Top Stories Today