Pharmacy and Poisons Board warns of fake diabetes drug circulating in market
The public is encouraged to report any encounters with such counterfeit products to the nearest healthcare facility or directly to the Pharmacy and Poisons Board.
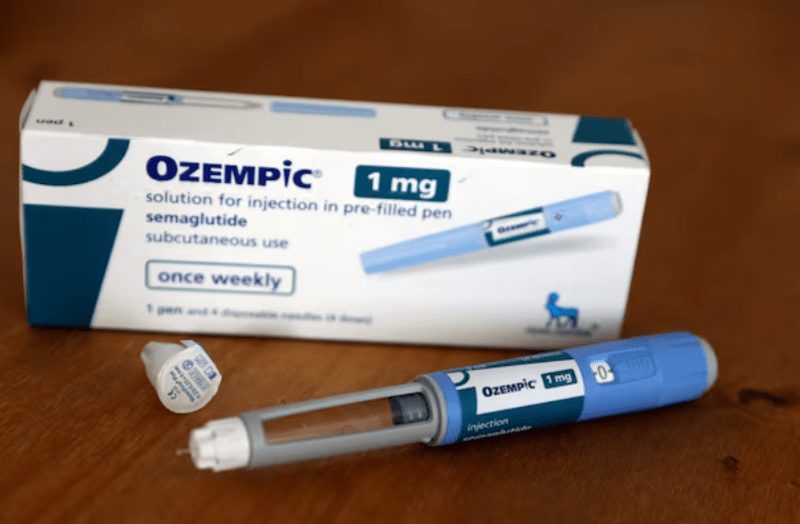
The Pharmacy and Poisons Board (PPB) has issued a public alert following a notice from INTERPOL regarding a counterfeit diabetes drug known as Ozempic Pens (Semaglutide).
"It has come to our attention that Apidra Solostar pens (glulisine), used to treat both type 1 and type 2 diabetes, have been falsely relabeled as Ozempic (Semaglutide) Pens," the notice stated.
More To Read
- Convenient but dangerous: The hidden health risks of ultra-processed foods
- Artificial sweeteners linked to higher diabetes risk, study finds
- Two weeks without sugar: What I learned about my body, culture and cravings
- KMPDC launches 2026 licence renewal for doctors, health facilities
- Pharmacy and Poisons Board flags falsified batch of Avastin (Bevacizumab 100 mg)
- Pharmacy board dismisses claims of medicine import ban, says drug supply uninterrupted
The PPB stressed that Ozempic Pens are not registered or authorised for distribution in the Kenyan market.
"Any product marketed as Ozempic Pens is illegal, and the Board cannot guarantee its safety, quality, or effectiveness."
In response, the PPB has launched rapid response measures and increased surveillance to determine if these counterfeit Ozempic Pens are circulating in Kenya.
The PPB warned the public and healthcare professionals against trading, distributing, wholesaling, retailing, issuing, dispensing, using, or administering the falsified Ozempic Pens, citing significant health risks.
The board urged the public and healthcare professionals to report any information regarding Ozempic Pens immediately.
"We remain committed to protecting public health and encourage vigilance in identifying and reporting any suspected sub-standard or falsified health products, as well as adverse drug reactions," the PPB stated.
The public is encouraged to report any encounters with such counterfeit products to the nearest healthcare facility or directly to the Pharmacy and Poisons Board.
Top Stories Today











































